is ionization endothermic or exothermic
This is really just the process of ionization. Unlike ionization energies which are always positive for a neutral atom because energy is required to remove an electron electron affinities can be negative.

Ionization Energy Scaffolded Notes By Threefourthsme Tpt Ionization Energy Scaffolded Notes Electron Configuration
Example of IE 1 of Magnesium.

. Classify Each Change Of StateMelting Freezing Vaporization Condensation Sublimation Deposition Ionization De-ionization As Endothermic Or Exothermic. Ionization is the process of removing negatively charged electrons from Why can electron affinity be positive or negative. Here is how to classify the phase changes as endothermic or exothermic. Electron affinity of chlorine exothermic Clg Cl g exothermic 5.
If we increases the temperature Kw increases and the equilibrium shifts towards the products. Ionisation energies are always endothermic The positive nucleus is attracted to the negative electron. Dissociation of the chlorine molecule Cl2g 2Clg exothermic 3. Hence it is positive.
Energy must be input in order to raise the particles up to the higher energy level. Ionization of rubidium Answer Bank Rbg Rbt g e- endothermic endothermic 4. The third term indicates lattice enthalpy which is exothermic and has a negative value. Why is this contradiction.
I dont get how ionization is an endothermic reaction. As we need a lot of heat to remove out the electron ionization energy is endothermic. For an endothermic reaction positive H an increase in temperature shifts the equilibrium to the right to absorb the added heat. ΔH is determined by the system not the surrounding environment in a reaction.
Similarly the second ionization energy will be the energy needed to expel the second electron. The autoionization of water is an endothermic reaction. Is the self ionization of water endothermic or exothermic. IE 1 stands for the first ionization energy.
Energy output Exothermic. Formation of RbCl from its ions Rbg CIg RbCls exothermic The overall. If we increases the temperature K w increases and the equilibrium shifts towards the products. An endothermic process absorbs heat and cools the surroundings.
Electrons are attracted to the nucleus therefore energy is needed to remove them. The ionization constant for water K w is 29 10 14 at 40 C and 93 10 14 at 60 C. The energy the atom requires to expel the first electron from its orbital. Correspondingly a temperature increase would favour the endothermic heat absorbing direction and a temperature decrease would favour the exothermic heat releasing direction.
The ionization constant for water K w is 29 10 14 at 40 C and 96 10 14 at 60 C. In general then you supply energy to eject an electron and thus it is an endothermic process regardless of the identity of M or A. Energy input Endothermic. Learn this topic by watching Ka and Kb Concept Videos.
Electrons are attracted to the nucleus therefore energy is needed to remove them. Note that ionization can be a part of more complex overall process that can be. Why is ionization exothermic. My understanding from Le Chateliers principle is that a rise in temperature adds heat to the system and a drop in temperature removes heat from the system.
Because an increase in temperature favors the endothermic side the products in this case we can conclude that the forward reaction is endothermic and not exothermic. The ionization energy is the energy required to completely remove a valence electron from a gaseous atom or ion. Because an increase in temperature favors the endothermic side the products in this case we can conclude that the forward reaction is endothermic and not exothermic. Endothermic reactions result in an overall positive heat of reaction q_rxn 0.
In an endothermic Reactions. Now since we require an energy input to perform this removal then we consider this process as endothermic. Ionization is the process of removing negatively charged electrons from Is the first ionization energy exothermic. An exothermic process releases heat causing the temperature of the immediate surroundings to rise.
Thus bond breaking is still endothermic which is dissociation but for ionization you are breaking and forming bonds and forming the bond is more exothermic than breaking it so you have an overall exothermic process. The autoionization of water is an endothermic reaction. Based on the above definition lets pick a few examples from our daily lives and categorize them as endothermic or exothermic. 1st Electron Affinity is usually exothermic as the energy released when the nucleus attracts the the additional electron is larger than the energy absorbed to overcome inter-electronic repulsion.
Sublimation of rubidium Rbs Rbg endothermic 2. Solution Because K w increases with increasing temperature the reaction in endothermic. Hence all ionization energies are positive. The ionization energy is the energy required to remove an electron or if you prefer the weakest and loosest electron in order to form a cation.
The second term indicates electron affinity which is exothermic and has a negative value. Begingroup Ionization is always an endothermic process as bound electrons have lower energy than free electrons. Ionization energy is endothermic because it requires an energy input to occur. For an exothermic reaction negative H an increase in temperature shifts the equilibrium to the left.
Exothermic and endothermic reactions cause energy level differences and therefore differences in enthalpy ΔH the sum of all potential and kinetic energies. N 2 g 3H 2 g 2NH 3 g. You need to put energy in to pull them apart. My thoughts are that.
The reactants have less potential energy than do the products. Therefore Ionization energy is an endothermic process because we need to supply energy to remove that outermost electron. Mgg - Mg g e-I 1 738 kJmol. When the temperature of the system is increased the reaction shifts toward the product side making.
Where the appropriate phase applies to the neutral A and A is a nonmetal. Exothermic vs endothermic process. It is very analogical to astronomical bodies bound by gravity of the central body. Ionization Energy has positive values because energy is always required to remove an electron it is endothermic.
The first term indicates the ionization energy which is endothermic and has a positive value. Is the self ionization of water endothermic or exothermic. Hence it is positive. For example in the equilibrium equation.
Identify each step as endothermic or exothermic.
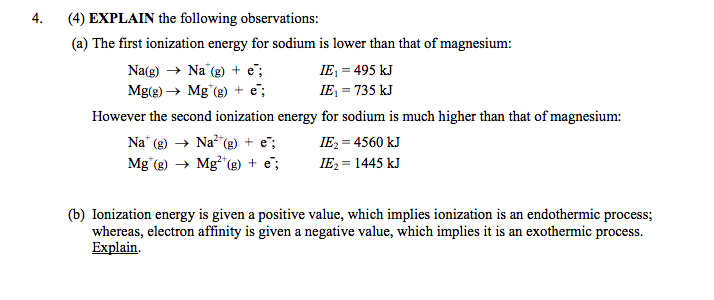
Solved 4 4 Explain The Following Observations A The Chegg Com

Login To Your Benchprep Account Teaching Chemistry Chemistry Lessons Chemistry Education

Solved Use The Ionization Energies And The Electron Affinities Given Below To Determine Whether The Following Reaction Is Endothermic Or Exothermic Mg G 2 Flg Mg2t G 2 F G Mg G Mgt G Mgt G Mg T G

Endothermic Exothermic Reactions Cornell Doodle Notes Distance Learning Doodle Notes Note Taking Strategies Exothermic Reaction

Electron Affinity Scaffolded Notes By Threefourthsme Tpt Electron Affinity Electron Configuration Ionization Energy

Endothermic Exothermic Cornell Doodle Notes By Sunrise Science Teachers Pay Teachers Doodle Notes Note Taking Strategies Exothermic Reaction
Posting Komentar untuk "is ionization endothermic or exothermic"